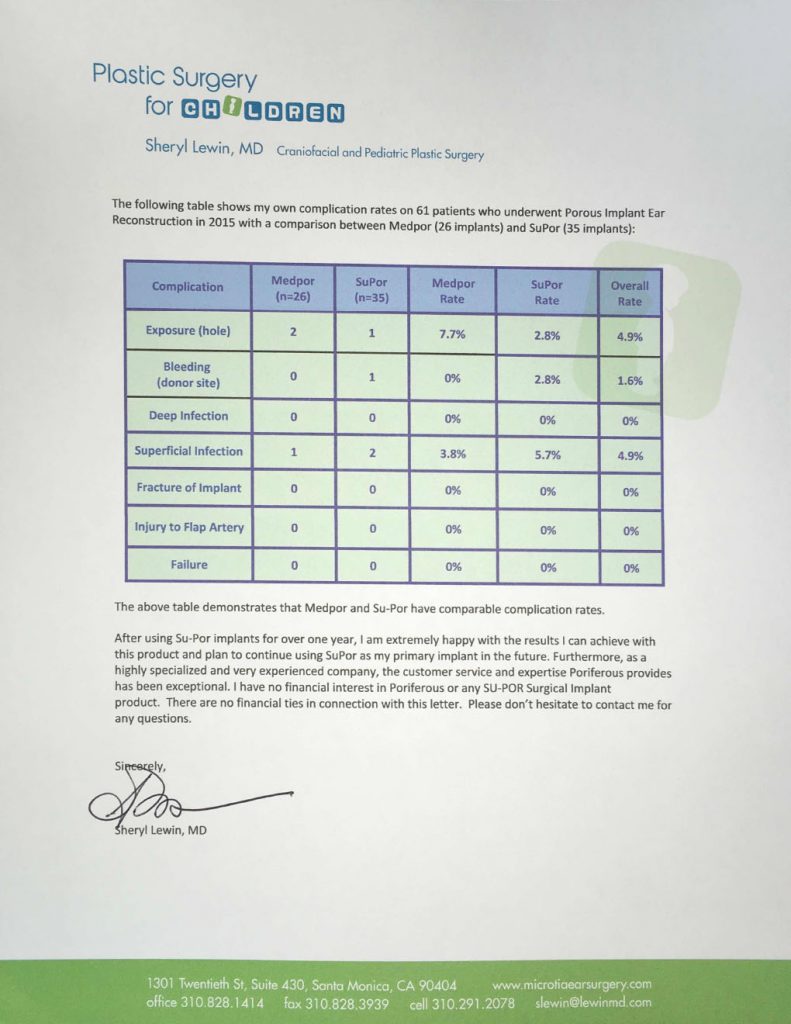
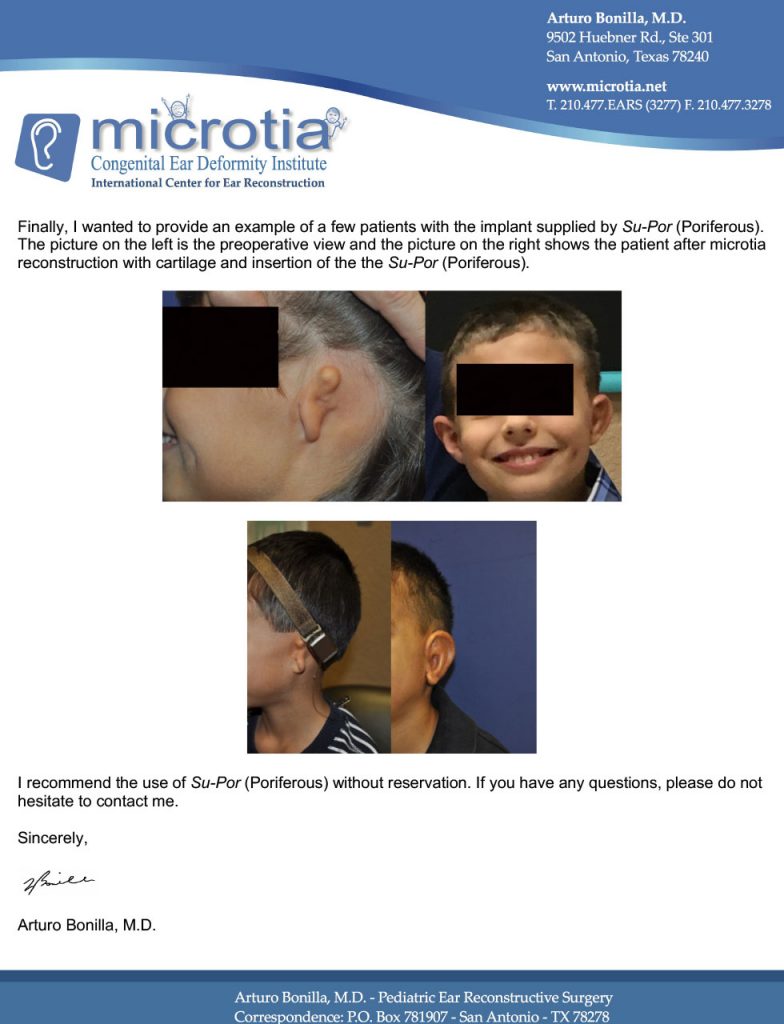
We Are Poriferous
Poriferous, LLC’s Mission
Poriferous continually innovates the process of CMF implant product development to provide a safe product that yields superior performance at a reasonable cost. We evaluate, meet, and exceed surgeons’ needs for a product that delivers outstanding results and services. Our implants optimize and safeguard surgeon and hospital time and resource investments. Poriferous remains an agile leader in the industry. We offer a service that is transparent, reasonably-priced, and ensures that doctors have the best tools to optimize their care of their patients.
What Is Su-Por?
Su-Por Surgical Implants are long-term implantable medical devices for the augmentation and restoration of the craniomaxillofacial area. We produce them from pure porous high-density polyethylene, the gold standard of polymer biocompatibility.
The devices are available in sheets, blocks, spheres, and anatomical shapes. The porous structures’ unique nature allows for the patients’ own fibrovascular to integrate, eliminate migration, reduce infection, and improve clinical performance.
Why Tissue Integration Is Important
Unlike non-porous implants, Su-Por biomaterial allows the patients’ immune system to effectively prevent and eliminate potential infections around and throughout the porous structure. Clinical evidence of Su-Por has proven to be superior to non-integrating implants. It is often cited as the ideal material for notoriously difficult revision procedures where non-porous devices required removal, often leaving damaged and scarred tissue.
Poriferous History
- 2011- CEO Develops Porous Polyethylene Implants
- 2012- Poriferous, LLC Founded
- 2014- Su-Por Surgical Implants Cleared by FDA 510(k) 140437
- 2015- ISO13485 Certification and CE Mark
- 2016- Su-Por Patient Specific Surgical Implants Cleared by FDA 510(k)152463
- 2016- Health Canada Medical Device License
- 2017- Poriferous Issued USA Patent 9636202 Support for Ear Base
- 2017- Poriferous Issued USA Patent 9724198 Orbital Floor Implant
- 2018- Received MDSAP Certification
- 2020- Received Notification of Intent to Grant European Patent for Cor-Tec
- 2020- U.S. Patent Issues Notice of Allowance for Channel Implant Patent
- 2020- Received Manufacturing Practices Certification
- 2021-Su-Por Global Presence
Frequently asked questions about Su-Por® surgical implants.
Testimonials
John Nguyen, MD Su-Por Quadro-Port Conical Implant